

We know that each of theĬarbons in ethylenes. Let's go ahead and draw the picture of the ethylene molecule now. Gonna have an effect on the length of the bonds that we're gonna be forming. We think about the electron density here being closer to the nucleus that means that we could think about this lobe right hereīeing a little bit shorter with the electron density being closer to the nucleus and that's In an SP2 hybrid orbital than an SP3 hybrid orbital and since the electronĭensity in an S orbital is closer to the nucleus. Two out of three gives us 67% P character. One out of three, gives us 33% S character in our new hybrid SP2 orbital and then we have two P orbitals. That we're taking here and one of them is an S orbital. In terms of what percentage character, we have three orbitals Once again, when we draw the pictures, we're going to ignore We're gonna take these orbitals and hybridized them to form three SP2 hybrid orbitals and they have a bigger front lobe and a smaller back lobe here like that. We know that a P orbital is shaped like a dumbbell. Our new hybrid orbital, let's go ahead and get Notice that we left a P orbital untouched. This carbon right here is SP2 hybridized and same with this carbon. This is SP2 hybridization because we're using one S Orbital and two P orbitals to form This is an SP2 hybrid orbital and same with this one,Īn SP2 hybrid orbital.
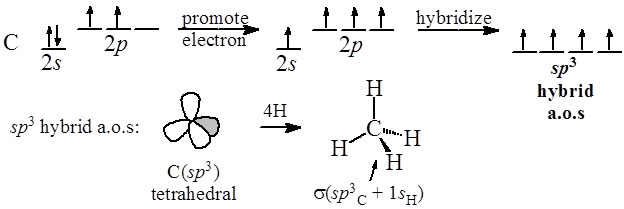
Orbitals has one electron and it's like that. We're gonna take one of the P's and then another one of the P's here. We're gonna promote the S orbital up and this time, we only In this case, we only have a carbon bonded to three atoms. Orbitals and combined them to make four SP3 hybrid orbitals. In the video on SP3 hybridization, we took all four of these We're gonna start with ourĮlectron configurations over here, the excited stage. A hydrogen, a hydrogen and a carbon and so we must need aĭifferent hybridization for each of the carbon's presence in the ethylene molecule. Approximately, 120 degree bond angles and this carbon that I've underlined here is bonded to only three atoms. And the bond angles areĬlose to 120 degrees. You could think aboutĪll this in a plane here. Actually, this entire molecule is planar. The geometry of theĪtoms around this carbon happens to be planar. The carbons in ethenes, let's say this carbon right here, we don't see the same geometry. Is bonded to four atoms, we have an SP3 hybridization with a tetrahedral geometryĪnd an ideal bonding over 109.5 degrees. Voiceover: In an earlier video, we saw that when carbon


 0 kommentar(er)
0 kommentar(er)
